Ultrasound in Prostate Disease: First-Line Imaging for Diagnosis, Biopsy Guidance, and Therapy
Introduction
Prostate diseases – ranging from benign prostatic hyperplasia (BPH) to prostate cancer – are among the most prevalent conditions affecting men worldwide. Prostate cancer is the second leading cause of cancer death in men; in the United States alone, an estimated 300,000 new cases and 35,000 deaths occurred in 2024. BPH, a non-malignant enlargement of the prostate, is extremely common in aging men and can significantly impact quality of life. Given the high prevalence of these conditions, accurate and timely diagnosis is critical for early intervention.
Ultrasound has long been a cornerstone in the evaluation of prostate disease. Conventional transrectal ultrasound (TRUS) emerged in the 1980s as a first-line imaging modality for the prostate, owing to its availability, real-time visualization, and ability to guide biopsies. Unlike modalities such as MRI or CT, ultrasound is cost-effective, widely accessible, and does not require ionizing radiation or a large infrastructure. These advantages make ultrasound an attractive initial imaging technique in many healthcare settings, especially in the U.S. and globally where access to advanced imaging may be limited. Indeed, TRUS-guided biopsy is still the standard confirmatory test after an elevated prostate-specific antigen (PSA) or abnormal digital rectal exam (DRE) in routine practice.
However, conventional ultrasound has well-recognized limitations in prostate cancer detection – many tumors are not conspicuous on grey-scale imaging, leading to modest sensitivity and specificity on the order of ~40–50%. In recent years, there has been a push to improve prostate imaging. Multiparametric MRI (mpMRI) has demonstrated higher sensitivity (86–92%) for detecting clinically significant cancer and is now recommended as a first-line diagnostic test in some countries (e.g. the UK) before any biopsy. Yet MRI has drawbacks – it is expensive, time-consuming, not suitable for certain patients (e.g. those with pacemakers or severe claustrophobia), and not universally available.
Fortunately, ultrasound technology is rapidly evolving to bridge this gap. New advancements – including ultrasound elastography, contrast-enhanced ultrasound with microbubbles, and high-frequency “micro-ultrasound” – are enhancing the diagnostic accuracy of ultrasound for prostate lesions. These innovations, often combined as multiparametric ultrasound (mpUS), have shown promise in detecting clinically significant prostate cancer with sensitivities approaching those of MRI. At the same time, ultrasound remains indispensable for guiding prostate biopsies and facilitating minimally invasive therapies. Techniques such as MRI–ultrasound fusion biopsy marry the superior tumor localization of MRI with the practical real-time guidance of ultrasound. Therapeutically, high-intensity focused ultrasound (HIFU) is an FDA-approved, non-invasive treatment for localized prostate cancer that uses ultrasonic energy to ablate tumor tissue, and newer methods like MRI-guided transurethral ultrasound ablation (TULSA) and Aquablation further expand ultrasound’s role in treatment.
This comprehensive review delves into the diagnostic and therapeutic applications of ultrasound in prostate disease. We will discuss the use of ultrasound as a first-line diagnostic tool in prostate cancer and BPH, the role of ultrasound in guiding prostate biopsies (with and without MRI fusion), and cutting-edge ultrasound technologies such as elastography, microbubble contrast, and ultra-high-frequency imaging. We will also compare ultrasound with other imaging modalities (MRI, CT) in prostate evaluation, emphasizing a global perspective (primarily U.S. practices with notes on other countries). The aim is to provide sonographers, radiologists, urologists, and other clinicians with an up-to-date, evidence-based perspective on how modern ultrasound can be optimally utilized in prostate disease.
Ultrasound Techniques for Prostate Imaging
Transrectal Ultrasound (TRUS): The workhorse of prostate imaging is transrectal ultrasound. In a TRUS exam, a high-frequency ultrasound probe (typically 6–12 MHz) is inserted into the rectum to obtain detailed images of the prostate gland. TRUS provides real-time, two-dimensional visualization of the prostate’s anatomy, including the peripheral zone (where ~70% of cancers occur), transition zone (common site of BPH nodules), central zone, and the prostatic capsule. On gray-scale TRUS, the normal peripheral zone is relatively homogeneous and is slightly hypoechoic (darker) compared to surrounding tissues. Prostate cancers classically appear as focal hypoechoic lesions in the peripheral zone on B-mode ultrasound. In fact, historically 60–80% of prostate cancers were noted to be hypoechoic on TRUS. However, up to 30–40% of cancers are isoechoic (indistinguishable from normal gland tissue on gray-scale) and a small fraction (~1–2%) are even hyperechoic. This means a substantial number of cancers can be “invisible” on conventional TRUS images. Furthermore, benign conditions like prostatitis, fibrosis, or calcifications can also produce hypoechoic areas, so a hypoechoic lesion on TRUS is not specific for cancer – only 17–57% of ultrasound-visible lesions actually turn out to be malignant on biopsy. Thus, the diagnostic accuracy of gray-scale TRUS alone is limited, with reported sensitivity and specificity as low as ~40–50% for cancer detection in prospective studies.
Despite these limitations in cancer detection, TRUS is invaluable for guiding biopsies and other interventions. A standard TRUS exam can measure the prostate’s volume (using ellipsoid formula dimensions), evaluate the gland’s contour and symmetry, and detect gross abnormalities (such as a hypoechoic tumor or cyst). TRUS also visualizes surrounding structures – the seminal vesicles, bladder base, and rectal wall – which helps in staging and procedural planning. Notably, TRUS is the primary imaging used to guide systematic prostate biopsy procedures (discussed in detail later). It is also used intraoperatively to guide needle placement for brachytherapy (radioactive seed implantation) and for monitoring ablative treatments like cryotherapy or HIFU.
Transabdominal Ultrasound: In addition to TRUS, a transabdominal (suprapubic) ultrasound approach can image the prostate through a filled urinary bladder acting as an acoustic window. Transabdominal ultrasound is noninvasive(external), but it provides much less detail of the prostate internal architecture due to greater depth and lower frequency probes. Its main use is to estimate prostate size (volume) and check for bladder outlet obstruction sequelae. For example, in men with BPH and lower urinary tract symptoms, a quick transabdominal scan can assess prostate enlargement, measure bladder wall thickening or trabeculation, and determine the post-void residual (PVR) urine volume. Guidelines generally recommend assessing prostate size and PVR in the evaluation of BPH, and ultrasound (either transrectal or transabdominal) is a preferred modality for this purpose. Transabdominal ultrasound is also helpful to detect hydronephrosis (kidney swelling) that could result from chronic urinary retention due to prostate obstruction.
Transperineal Ultrasound: Another ultrasound technique uses a perineal approach (through the skin between the scrotum and anus) to visualize the prostate. This is less common for diagnostic imaging but is increasingly used for guiding prostate biopsies. With specialized transperineal probes or using a transrectal probe placed against the perineum, the prostate can be imaged in sagittal and axial planes. The transperineal route avoids traversing the rectum and thus virtually eliminates the risk of infection when used for biopsy. It also provides good access to the anterior prostate regions. However, transperineal imaging typically requires working in an operative setup (often under local or general anesthesia for biopsy), whereas TRUS is more easily performed in outpatient clinics for diagnostic scanning. Overall, each ultrasound approach – transrectal, transabdominal, transperineal – has its niche, but TRUS remains the primary method for dedicated diagnostic prostate imaging and for guidance during transperineal prostate biopsies due to its optimal balance of image quality and practicality.
Ultrasound in the Diagnosis of Prostate Cancer
Ultrasound is often the first imaging test employed when prostate cancer is suspected. The traditional diagnostic pathway in the U.S. has been: an abnormal PSA and/or DRE prompts a TRUS-guided biopsy to obtain tissue confirmation. While this paradigm has undoubtedly helped detect cancers earlier and reduce mortality, it has limitations in sensitivity and specificity. Here we review how well ultrasound performs in detecting prostate cancer, and how newer techniques are improving its diagnostic yield.
Gray-Scale and Doppler Ultrasound Findings
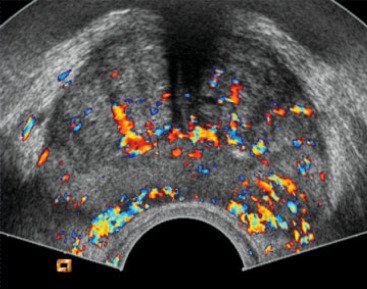
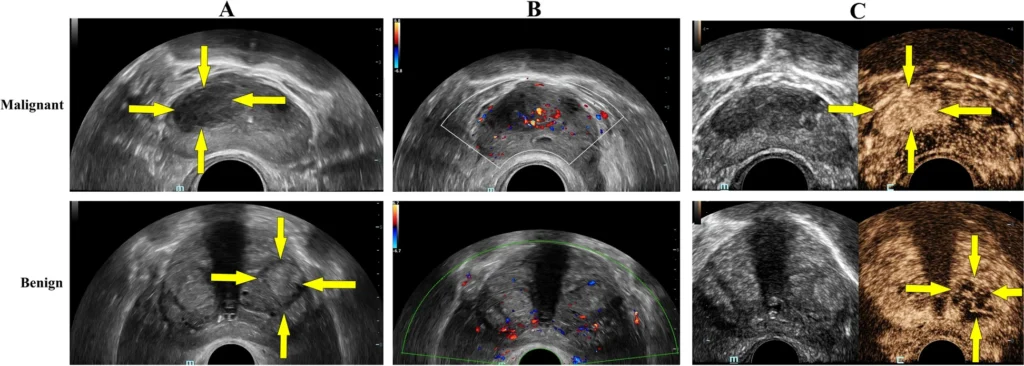
On conventional gray-scale TRUS, as noted, the classic appearance of prostate carcinoma is a hypoechoic nodule in the peripheral zone. When such a lesion is seen, especially if irregular and in the setting of elevated PSA, suspicion for cancer is high. TRUS can also hint at extracapsular extension of cancer if features like a bulging capsule or breach of the prostate outline are observed adjacent to a lesion. However, many clinically significant cancers do not produce a distinct hypoechoic signature. Tumors can be isoechoic or obscured by heterogeneous gland texture in men with coexistent BPH nodules or inflammation. The limited intrinsic contrast of soft tissues in B-mode ultrasound means that small or infiltrative tumors often blend into the background. As a result, the sensitivity of gray-scale TRUS for prostate cancer is only around 37–55% in systematic reviews. In other words, TRUS misses nearly half of cancers if used as the sole diagnostic tool. Its specificity is somewhat higher (often 70–90% in some series when strict criteria are used) because normal gland tissue usually appears homogeneous – but specificity suffers when benign conditions mimic cancer. For example, chronic prostatitis or scars can also appear as hypoechoic areas. Overall, conventional ultrasound alone cannot reliably distinguish cancerous from benign tissue in a significant fraction of cases. One study summarized that TRUS imaging has “very limited” sensitivity and specificity (40–50%) for prostate cancer detection, and even adding color Doppler only marginally improves these metrics. Color and power Doppler ultrasound can reveal hypervascular areas within the prostate – increased blood flow that might occur in tumors due to neoangiogenesis. In some cases, a prostate cancer will show a focal increase in vascularity on Doppler imaging, raising suspicion. However, many small tumors have slow or sparse flow that Doppler cannot resolve, and benign processes (like inflammation or BPH) can also show increased blood flow. Thus, Doppler alone is not sufficiently sensitive for reliable cancer detection. It does, however, complement gray-scale imaging and has been used to target biopsies to vascular “hot spots” with some success.
Given these diagnostic shortcomings of basic ultrasound, it’s not surprising that in the past decade MRI has become the preferred imaging for detecting prostate cancer. Multiparametric MRI – which combines anatomical T2-weighted images with diffusion-weighted imaging and often contrast enhancement – can identify suspicious lesions with much higher sensitivity (reported 86–93%) and moderate specificity (~60–80%). In 2019, the UK’s National Institute for Health and Care Excellence (NICE) updated its guidelines to recommend mpMRI as the first-line investigation for men with suspected localized prostate cancer, prior to any biopsy. This change, driven by trials like PROMIS and PRECISION, was shown to reduce unnecessary biopsies in men with low-risk findings and improve detection of clinically significant cancers. Similarly, European Association of Urology (EAU) guidelines and American Urological Association (AUA) guidelines now strongly consider MRI before biopsy in many patients.
Nonetheless, MRI is not universally available or suitable for all patients (e.g., those with certain implants or severe claustrophobia). Moreover, MRI can still miss up to 10–15% of significant tumors (especially small ones) and has notable interobserver variability in interpretation. Therefore, ultrasound remains highly relevant as an initial imaging modality and as a necessary accompaniment to biopsy. The good news is that ultrasound has not remained static – advanced ultrasound techniques are making it more competitive with MRI in cancer detection, as discussed in a later section. Modern “multiparametric ultrasound” approaches that combine gray-scale, Doppler, elastography, and contrast enhancement have shown sensitivities in the 70–80% range for significant cancer, narrowing the gap with MRI.
In summary, conventional TRUS by itself is imperfect for diagnosing prostate cancer. It often cannot confirm a cancer non-invasively – hence tissue biopsy has been required. However, TRUS does play an essential role in the diagnostic pathway by enabling systematic sampling of the prostate. And with new enhancements (like elastography and contrast ultrasound), its diagnostic accuracy is improving. Importantly, ultrasound is real-time and in-office, allowing the urologist or radiologist to immediately investigate a PSA elevation without the logistics of MRI. Especially in resource-limited settings or for patients who cannot undergo MRI, ultrasound is the first-line imaging tool to evaluate the prostate.
Ultrasound in Benign Prostatic Hyperplasia (BPH) and Other Benign Conditions
While much attention is given to cancer, ultrasound is equally important in assessing benign prostate conditions such as BPH. BPH is an extremely common condition – by age 60, roughly half of men have histologic BPH, and prevalence reaches 80–90% by age 80. BPH can lead to prostate enlargement, bladder outlet obstruction, and lower urinary tract symptoms (LUTS) like weak stream and nocturia. Ultrasound is the modality of choice for evaluating prostate size, which is a key factor in BPH management.
Using either TRUS or transabdominal ultrasound, the prostate’s dimensions can be measured and volume calculated. Volume correlates with symptom severity and guides treatment selection – for example, prostates larger than ~80 ml might be better served by surgical enucleation or newer techniques like Aquablation rather than standard TURP. Clinical guidelines (AUA 2021) advise that in men with LUTS possibly due to BPH, an assessment of prostate size (via ultrasound or other imaging) should be considered, especially prior to any surgical intervention. Ultrasound also measures post-void residual urine efficiently, which indicates how much obstruction is present (a high residual volume suggests significant bladder emptying difficulty). A PVR is recommended as part of the BPH work-up. Additionally, ultrasound of the kidneys can check for hydronephrosis (swelling of kidneys) as a complication of chronic bladder outlet obstruction, and ultrasound of the bladder can reveal diverticula or stones that result from long-standing BPH.
On TRUS, BPH classically appears as an enlarged transition zone with heterogeneous echotexture. BPH nodules can be of mixed echogenicity; some are hypoechoic and might superficially resemble tumors. In general, however, BPH changes are in the central gland (transition zone), often cause a layered appearance (with an “inner gland” expansion), and may show calcifications or cystic changes (common in benign nodules). TRUS helps distinguish a benign median lobe enlargement (which can protrude into the bladder) from other pathology. It’s worth noting that BPH nodules themselves are firmer than normal tissue – they can cause the gland to feel hard on DRE and they elevate PSA due to increased tissue volume and perhaps vascularity. These factors can confound cancer detection: a man with BPH may have a high PSA and a large, nodular prostate on TRUS with hypoechoic areas, yet no malignancy. Advanced ultrasound techniques like elastography (stiffness mapping) might help differentiate BPH from cancer, since cancer tissue tends to be more uniformly stiff whereas BPH nodules can have variable consistency. We will discuss elastography in detail later.
Ultrasound is also valuable in diagnosing and managing prostatic abscesses and prostatitis. In cases of refractory bacterial prostatitis or abscess, TRUS can visualize fluid collections within the prostate. An abscess appears as an anechoic or hypoechoic cavity, sometimes with internal echoes (debris). TRUS can guide aspiration or drainage of prostatic abscesses by directing a needle into the collection through either the rectal wall or perineum. This minimally invasive approach has high success in resolving infections and is far less morbid than surgical drainage. For example, one series noted that TRUS-guided needle aspiration of prostate abscesses achieved complete resolution in the majority of patients with low complication rates. Thus, in the context of acute or chronic prostatitis with suspected abscess, ultrasound is the first imaging to perform and often doubles as the treatment tool.
In summary, for benign prostate conditions (BPH, prostatitis, abscess), ultrasound is usually the first and often the only imaging modality required. It provides crucial information on gland size and anatomy that guide management of BPH. It also facilitates interventions such as abscess drainage or bladder catheter placement in retention. And while benign changes can sometimes mimic cancer on ultrasound, correlation with clinical factors (PSA, symptoms) and judicious use of biopsy or adjunct imaging can clarify the diagnosis. The key point is that ultrasound is not just for cancer – it is an all-purpose imaging technique for a variety of prostate diseases due to its safety, convenience, and multipurpose utility.
Ultrasound-Guided Prostate Biopsy: TRUS vs MRI Fusion
Tissue diagnosis via biopsy is the gold standard for confirming prostate cancer. Ultrasound has been indispensable in this realm as the guidance method for biopsy needles. Over the years, prostate biopsy techniques have evolved from blindly directed sextant biopsies to systematic sampling under TRUS guidance, and more recently to targeted biopsies using MRI-ultrasound fusion. In this section, we compare the traditional TRUS-guided biopsy approach with the newer MRI-targeted techniques, highlighting the strengths of ultrasound guidance in each.
Standard TRUS-Guided Systematic Biopsy
The concept of systematic prostate biopsy under ultrasound guidance was introduced in the late 1980s and quickly became the standard of care. In a typical TRUS-guided biopsy, the patient is placed in a lateral decubitus position, a transrectal ultrasound probe is inserted for visualization, and a spring-loaded biopsy needle device is used to take core samples of the prostate through the rectal wall. Originally, a sextant (6-core) scheme was used (sampling apex, mid, base on left and right). Over time, this was expanded to 10–12 cores (extended biopsy scheme) to improve cancer detection, since sextant biopsies missed a significant number of cancers. Contemporary systematic biopsy usually obtains 12 cores distributed through the peripheral zone bilaterally.
Under ultrasound, the physician can systematically map the biopsy locations: typically two cores each from the apex, mid gland, and base on each side, often with additional cores directed at any suspicious areas seen on TRUS. While the ultrasound may not clearly show all tumors, it ensures that the biopsy needle adequately covers the prostate regions. Without ultrasound guidance, prostate biopsy would essentially be blind. Studies have shown that systematic TRUS biopsy detects many cancers that would be missed by random or digitally guided biopsies. However, even with 12+ cores, sampling is limited – the procedure might only sample ~0.1% of the gland volume. Thus, there is a known false-negative rate: an initial TRUS-guided biopsy can miss significant cancer in ~20% of patients, necessitating repeat biopsies if clinical suspicion remains. In fact, the literature indicates a false-negative range of 17–21% for initial biopsy series. This means roughly one in five men with an initial “negative” biopsy may actually harbor cancer that was not hit by any needle, especially if the tumor is small or anteriorly located (areas less sampled by transrectal approach).
Another limitation of systematic biopsy is over-detection of indolent cancer. By sampling broadly, we sometimes find low-grade (Gleason 6) microfoci that might never pose a threat (overdiagnosis estimated at ~27–56% in PSA-era biopsies). These findings can lead to overtreatment. The ideal would be to preferentially find only clinically significant cancers (Gleason ≥7, larger volume) and not the tiny Gleason 6 lesions.
Despite these issues, standard TRUS-guided biopsy has undeniably improved early detection. It has been shown to reduce the incidence of advanced prostate cancer and has been partly credited (along with PSA screening) for the decline in prostate cancer mortality since the 1990s. Yet, due to the drawbacks mentioned, there has been a drive to refine biopsy techniques.
From an ultrasound standpoint, a few strategies were tried to improve yield: using Doppler ultrasound to target hypervascular zones, using saturation biopsy (taking >20 cores, often via transperineal template guided by ultrasound under anesthesia), and using contrast-enhanced ultrasound or elastography to target cores (discussed in next section). Saturation biopsies do increase cancer detection modestly, but at the cost of more cores (and more side effects). In fact, taking more than 12 cores has diminishing returns and can significantly increase bleeding or pain, so it’s not routine unless in repeat biopsy scenarios. Combining Doppler, elastography, or CEUS with systematic biopsy has shown improved detection rates of significant cancers by a few percentage points. For example, one study reported that adding CEUS-targeted cores to systematic biopsy increased cancer detection by ~2–8%, and adding elastography-targeted cores increased detection by ~7–15%. These incremental gains showed that imaging enhancements can help, but a bigger leap in biopsy accuracy came with the advent of MRI targeting.
It is also important to address the safety of TRUS biopsies. Complications include hematuria, hematospermia (blood in semen), rectal bleeding, pain, and infection. Most men will have some blood in urine or stool after biopsy, but it is usually self-limited. The most concerning risk is infection, particularly post-biopsy sepsis from rectal flora introduced into the prostate or bloodstream. Transrectal biopsy has a reported sepsis risk historically around 1–3% (and in earlier eras of less antibiotic resistance, lower, but rising with resistant E. coli). Modern practices use prophylactic antibiotics and bowel prep, but fluoroquinolone-resistant organisms have led to persistent infection risks. Transperineal biopsy (where needles go through the perineal skin under ultrasound guidance) has emerged as a safer alternative with regards to infection – multiple studies and meta-analyses report near-zero (<0.5%) sepsis rates with transperineal approaches. Many centers worldwide have shifted to transperineal systematic biopsy (often under local anesthesia) to virtually eliminate severe infections while still using ultrasound guidance (just via a template grid placed on the perineum). The trade-off is that transperineal procedures may require more setup and often anesthesia, but they offer more complete sampling of anterior zones and better safety. In the U.K. and Australia, for example, transperineal template biopsy is now common. In the U.S., transrectal biopsy remains prevalent in-office, but there is increasing adoption of transperineal biopsy devices. Regardless of route, ultrasound is the essential guiding modality for both techniques – it allows visualization of needle trajectory and prostate location in real time.
In summary, the standard TRUS-guided biopsy (12-core systematic) is an ultrasound-driven procedure that has been the backbone of prostate cancer diagnosis. It is effective but not perfect, leading to both missed cancers and detection of insignificant ones. Ultrasound’s role here is to facilitate systematic sampling, and improvements in ultrasound targeting (Doppler, CEUS, elastography) have provided modest benefits.
MRI-Ultrasound Fusion Targeted Biopsy
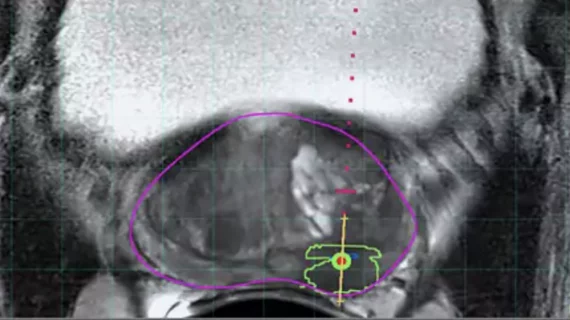
A significant advancement in the last decade has been the incorporation of pre-biopsy MRI information to target biopsies to suspicious lesions, rather than relying on random sampling. MRI-ultrasound fusion biopsy combines the strengths of both imaging modalities: the superior lesion detection of MRI with the convenience and real-time guidance of ultrasound.
The workflow typically involves the patient getting a multiparametric MRI of the prostate before biopsy. If the MRI shows one or more suspicious lesions (assigned PI-RADS scores), these can be targeted. There are two main approaches:
- Cognitive fusion biopsy: The operator (urologist or radiologist) performs a TRUS biopsy while mentally projecting where the MRI lesion is located in the prostate. For example, if MRI shows a lesion in the anterior horn of the peripheral zone at mid-gland on the right, the operator will aim the ultrasound-guided needle to that approximate location. This method requires skill and spatial understanding, but no special software. It has a learning curve and somewhat variable accuracy.
- Software-based MRI-US fusion biopsy: Here, dedicated fusion platforms/software are used. The MRI images (with contoured lesions) are loaded into an ultrasound machine or an attached computer. During the TRUS exam, the system electronically or mechanically fuses the MRI and real-time ultrasound by coregistration (often by aligning prostate contours or using fiducial markers). This allows the MRI targets to be superimposed on the ultrasound screen. The operator can then direct the biopsy needle precisely into the MRI-defined lesion. Fusion devices often use electromagnetic tracking or stepper motors to know the ultrasound probe position in space, enabling accurate alignment. This approach is more technologically complex but can yield very precise targeting within a few millimeters of the intended target.
Several prospective studies and randomized trials have validated the benefit of MRI-targeted biopsy. The landmark PRECISION trial (2018) showed that in biopsy-naïve men, using MRI first and doing targeted biopsies (omitting systematic biopsy if MRI was negative) detected more clinically significant cancers (38% vs 26%) and fewer insignificant ones compared to the standard 12-core approach. Other studies have shown that targeted biopsies can improve the detection of high-grade cancer by ~30% and reduce detection of low-grade cancer by ~17%, relative to systematic biopsy. MRI-targeted cores also tend to better predict the true grade of tumors (improving concordance with final pathology) because they sample the heart of the lesion, whereas systematic cores might hit edges.
However, MRI-targeted biopsy is not foolproof. MRI misses some lesions, and some cancers (especially in the anterior or apex) might not be clearly seen on MRI. For this reason, many protocols still combine targeted and systematic cores to maximize sensitivity. In fact, the combination of targeted + systematic finds the most cancers (each method can miss some that the other finds). One analysis noted that targeted biopsy alone could miss up to 10–15% of clinically significant cancers that had no MRI correlate but were found on systematic sampling. Conversely, systematic biopsy might miss those MRI-visible lesions that targeted biopsy hits. Thus, the current optimal approach in many centers is a mixed strategy – do both targeted and systematic cores in the same session (often called a “fusion biopsy” or “combination biopsy”). This does increase the number of cores and cost, but ensures high sensitivity.
From the perspective of ultrasound’s role, it remains central. Even in MRI-fusion biopsy, the actual needle placement is done under ultrasound guidance. MRI by itself can be used in-bore for direct MRI-guided biopsy, but that is resource-intensive (requiring an interventional MRI suite) and time-consuming (each needle placement under MRI is lengthy). In practice, MRI-guided in-bore biopsies are rare; MRI–US fusion performed by ultrasound machines in clinic is far more common due to its efficiency. Fusion devices resolve the prior issue that MRI is not real-time – by linking MRI to ultrasound, the operator can see and fire at the target during an office procedure, which is a major workflow advantage. As Chen et al. summarized, “MRI–ultrasound fusion combines the high sensitivity of MRI with the practicality of ultrasound guidance”. It effectively boosts the yield of ultrasound-guided biopsy to what MRI can reveal.
It is worth noting that early evidence suggests using MRI-targeted biopsy leads to improved patient outcomes: higher rate of significant cancer detection, and potentially a reduction in unnecessary biopsies and overdiagnosis. Cost-effectiveness analyses (e.g., a 2024 Health Technology Assessment in the UK) have generally found that adding MRI (and doing fewer biopsies or targeted biopsies) is cost-effective in the long run. The global trend, especially in countries like the UK and in Europe, is to integrate MRI into the diagnostic algorithm for most men with elevated PSA prior to biopsy. In the U.S., adoption is increasing, though not yet universal (due to variable insurance coverage and MRI access in some communities). In places where MRI is not readily available, advanced ultrasound techniques (like contrast and elastography) or high-resolution micro-ultrasound (discussed next) are attractive alternatives to improve cancer detection without MRI.
Finally, a mention about micro-ultrasound targeted biopsy: A novel approach is using high-resolution 29 MHz transrectal micro-ultrasound to directly visualize suspicious lesions (with a scoring system called PRI-MUS) and target them, analogous to MRI targeting. Early studies show micro-ultrasound can detect significant tumors with sensitivity around 90% in experienced hands. This technology essentially aims to make ultrasound itself a targeting tool for biopsy, potentially obviating the need for MRI in some cases. We will detail micro-ultrasound later, but it’s part of the evolving landscape of ultrasound-guided targeted biopsy.
Key Point: The integration of MRI with ultrasound guidance (fusion biopsy) has markedly improved the diagnostic accuracy of prostate biopsies. Ultrasound remains the workhorse for guiding the biopsy needle, while MRI provides better lesion localization. Together, they represent a powerful synergy in prostate cancer diagnosis. As technology advances, ultrasound-based targeting (via micro-ultrasound or advanced US modalities) may further increase what ultrasound can accomplish on its own, keeping it at the forefront of prostate cancer evaluation.
Advanced Ultrasound Technologies in Prostate Imaging
One of the most exciting aspects of prostate imaging today is the development of advanced ultrasound techniques to augment or go beyond conventional gray-scale TRUS. These include ultrasound elastography, contrast-enhanced ultrasound (CEUS) with microbubbles, and ultra-high-frequency micro-ultrasound. By providing new types of tissue information – such as stiffness or vascularity at the microvascular level – these modalities are improving the ability of ultrasound to detect and characterize prostate lesions. When used together, some have dubbed this approach as multiparametric ultrasound (mpUS), drawing an analogy to mpMRI. In this section, we explore each of these technologies, their evidence base, and how they are applied in prostate disease.
Ultrasound Elastography in Prostate Disease
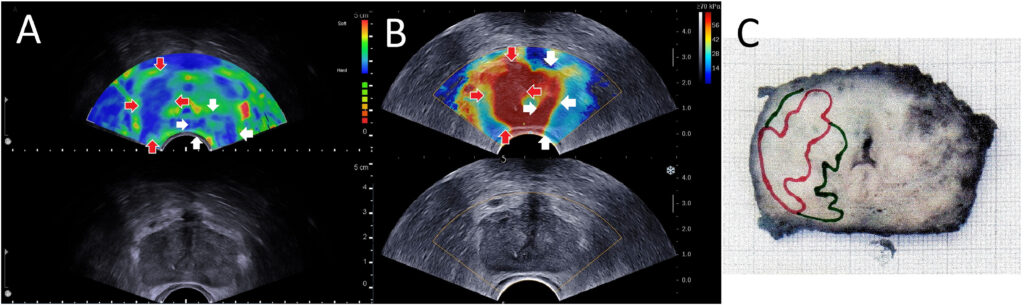
Elastography is an ultrasound technique that measures tissue stiffness. The rationale in the prostate is straightforward: cancerous tissue tends to be stiffer than normal prostate tissue due to factors like increased cell density, fibrosis (collagen deposition), and loss of normal glandular architecture. This is the same principle that makes a hard nodule on DRE concerning for cancer – but DRE is subjective and can only assess the gland’s posterior surface. Ultrasound elastography, by contrast, can create an internal “stiffness map” of the whole prostate in vivo, potentially highlighting hard lesions within the gland that might represent tumors.
There are two main types of ultrasound elastography used in the prostate: strain elastography and shear-wave elastography.
- Strain Elastography (SE): This technique involves applying slight pressure (compression) with the transrectal probe and measuring the resulting tissue deformation (strain). Harder tissue deforms less under compression than softer tissue. The ultrasound system computes relative strain and displays an elastogram – typically a color map overlaid on the B-mode image where, for example, blue might indicate stiff areas and red soft areas (or vice versa). It’s a qualitative or semi-quantitative method; often the stiffness of a region is compared to adjacent “normal” reference tissue or to the overall gland. Strain elastography can identify a stiff lesion (e.g., a cancer) as a focal area of low strain (hard) within the softer surrounding prostate. It requires a steady hand and often multiple compressions to get a usable image. Early studies of strain elastography in prostate cancer showed that it can improve cancer detection when used alongside TRUS, with reported sensitivity around 70–82% and specificity 60–95%. A meta-analysis by Zhang et al. found pooled sensitivity ~72% and specificity ~76% for strain elastography in detecting prostate cancer, using prostatectomy specimens as reference. These numbers are an improvement over conventional B-mode alone. Elastography tended to have particularly high negative predictive value – one study reported an NPV ~95–99% when no stiff areas were seen on elastography (suggesting a low chance of missing significant cancer). However, strain elastography has limitations: it provides relative stiffness (so results can vary with how compression is applied), and the presence of large BPH nodules or calcifications can affect readings. It also doesn’t give an absolute stiffness value.
- Shear-Wave Elastography (SWE): This is a more quantitative method. In SWE, the ultrasound probe emits a focused acoustic radiation force impulse (ARFI) into the tissue, which generates shear waves that propagate through the prostate. The speed of these shear waves is measured – stiffer tissue allows faster shear wave propagation. The result is typically an absolute measurement of Young’s modulus (kPa) or shear wave velocity (m/s) for each small region, which can be color-coded on an image. SWE provides a quantitative stiffness map without needing manual compression. For instance, a prostate cancer might show a stiffness of 80 kPa versus normal prostate ~30 kPa. Several studies have demonstrated the performance of SWE. For example, Boehm et al.found that using a cutoff of 50 kPa for suspect areas yielded a sensitivity ~81% and specificity ~69% for significant cancer (with higher stiffness correlating with higher Gleason grade). Another study reported that with a cutoff of 35 kPa, they achieved negative predictive value of 96% – meaning if the prostate had no area stiffer than 35 kPa, significant cancer was very unlikely. A 2019 meta-analysis (Yang et al.) concluded that SWE could be a “feasible technique to ameliorate PCa detection” and potentially reduce unnecessary biopsies. Furthermore, elasticity correlates with tumor grade: higher Gleason cancers tend to be stiffer. In one series, Gleason 6, 7, and ≥8 tumors had mean stiffness of ~92, 102, and 132 kPa respectively, showing a clear stepwise increase. This opens the possibility that elastography not only detects cancer but might help assess its aggressiveness noninvasively.
Elastography can be particularly helpful for cancers in the anterior fibromuscular stroma or deep transition zone, which are hard to visualize on B-mode but can be detected as focal stiff areas. It also may help distinguish benign stiff entities (like calcified BPH nodules) from cancer: BPH nodules can be firm but often not as uniformly stiff as cancer and have different elastographic patterns. However, overlap exists, and absolute diagnosis by elastography alone is not currently possible – it’s a complement to imaging and biopsy, not a replacement.
One challenge in implementing elastography widely has been the lack of standardization. Different machines have different scales and techniques (some use strain ratio calculations, others provide kPa values). There is also a learning curve to interpreting elastograms. Despite this, numerous studies validate that elastography significantly improves prostate cancer detection when added to TRUS, and can guide targeted biopsies. A recent (2025) review by Liang et al.concluded that ultrasound elastography has “significant advantages in diagnostic accuracy” for prostate diseases compared to conventional methods, though it noted the need for standardized protocols and broader availability of equipment. Elastography is also being investigated for treatment monitoring – for example, after focal therapy or radiation, elastography might detect residual stiff tumor versus softened treated tissue.
In summary, ultrasound elastography provides a virtual “finger” to palpate the prostate from the inside, mapping stiffness to highlight suspicious areas. It has shown improved detection of clinically significant cancers in many studies and is an exciting addition to the sonographer’s and urologist’s toolkit. As the technology matures and becomes more standardized, elastography could be routinely integrated into prostate ultrasound exams to decide where to biopsy or whether a patient can safely avoid biopsy (if the gland is entirely soft and unremarkable on elastography, for instance).
Contrast-Enhanced Ultrasound (CEUS) and Microbubble Imaging
Another major advance in ultrasound is the use of microbubble contrast agents to visualize blood flow and perfusion in the prostate. Tumors typically induce abnormal microvascular patterns – they often have increased blood flow and chaotic vessel architecture due to angiogenesis. Conventional Doppler ultrasound is not very sensitive to slow flow in small vessels, but microbubble contrast can overcome that limitation by enhancing the ultrasound signal from blood.
In CEUS, a contrast agent consisting of microscopic gas-filled bubbles (encased in a lipid or protein shell) is injected intravenously. These microbubbles circulate and reflect ultrasound strongly, allowing real-time visualization of blood perfusion in tissues. In the prostate, a malignant tumor will often demonstrate enhanced contrast uptake (appearing as a focal area of rapid contrast wash-in and wash-out) compared to normal tissue. By observing the contrast kinetics (usually over a few minutes after injection), one can generate Time-Intensity Curves (TIC) for regions of interest. Parameters like Time to Peak (TTP) enhancement, Peak Intensity, Wash-in rate, and Area Under Curve can be quantified. Tumors tend to show a shorter time-to-peak and higher peak intensity (reflecting quicker and greater filling due to neovascularity). For instance, a study by Zhu et al. found that higher grade prostate cancers had a significantly shorter contrast arrival time and time-to-peak, as well as a higher peak intensity than lower grade tissue. This makes intuitive sense: aggressive cancers have more leaky, high-flow vessels that light up earlier on contrast.
CEUS of the prostate is typically performed transrectally simultaneously with B-mode imaging. The microbubbles remain within the vasculature (they do not leak into interstitium), so what is seen is essentially a map of blood volume and flow in the prostate. A cancer might stand out as an “early and bright” enhancing focus. Some cancers, however, especially small or less angiogenic ones, might not show a big difference. Also, inflammation can cause increased blood flow and mimic tumor on CEUS. Nonetheless, multiple studies have shown CEUS-targeted biopsy improves cancer detection. As mentioned earlier, adding CEUS guidance to systematic biopsy increased cancer detection rates by a few percent in some trials. Mitterberger et al. (one of the early pioneers of prostate CEUS) reported that contrast-targeted biopsies could detect ~10% more cancers after an initial negative biopsy round. A 2017 meta-analysis found that CEUS-targeted biopsy had a higher detection rate for clinically significant cancer than systematic biopsy alone, though results varied by study. In absolute terms, sensitivity of CEUS in detecting prostate cancer lesions is around 59–81% with specificity ~70–88%according to a systematic review of parametric ultrasounds. These numbers indicate a notable improvement over unenhanced ultrasound, though not as high as MRI. CEUS particularly shines in identifying hypervascular tumors in the peripheral zone. In the transition zone, BPH nodules can also enhance, which may reduce specificity (one analysis noted CEUS sensitivity ~58% and specificity 70% in peripheral zone, versus 38% sensitivity, 79% specificity in the transition zone).
One variant of contrast imaging is Cadence Contrast Pulse Sequencing (CPS) or similar proprietary techniques that can even allow visualization of microbubble flow at the capillary level in real time. New research in “super-resolution ultrasound” can localize microbubbles with such precision that microvessels beyond the classical resolution of ultrasound are mapped. A 2022 study demonstrated that super-resolution ultrasound could map prostate tumor microvascular architecture and potentially differentiate cancerous patterns. This is still investigational but shows the potential of microbubble imaging to provide MRI-like or even histology-like vascular detail noninvasively.
CEUS does require an IV injection and adds a bit of time and cost, and a very small risk of allergic reaction (the microbubble agents are generally safe, with low incidence of side effects). It’s not yet standard for all prostate imaging, but many tertiary centers use CEUS especially in the setting of a prior negative biopsy and persistent suspicion, or to aid targeted biopsy. CEUS can also be used post-treatment – for example, after HIFU or focal laser ablation, a contrast ultrasound can assess the perfusion of the treated zone (a successfully ablated zone should have no contrast uptake, whereas residual viable tumor would enhance). This is a convenient way to follow up patients without repeated MRI or biopsies, although at present MRI is more commonly used for follow-up imaging.
In summary, contrast-enhanced ultrasound provides a dynamic assessment of prostate vascularity, which complements the anatomical imaging of B-mode. It improves detection of cancer by highlighting angiogenic lesions. CEUS is an important component of multiparametric ultrasound and will likely see increased use as more urologists and radiologists become comfortable with contrast agents. Its ability to improve targeting and potentially monitor treatment response makes it a versatile tool in prostate imaging.
High-Frequency “Micro-Ultrasound” Imaging
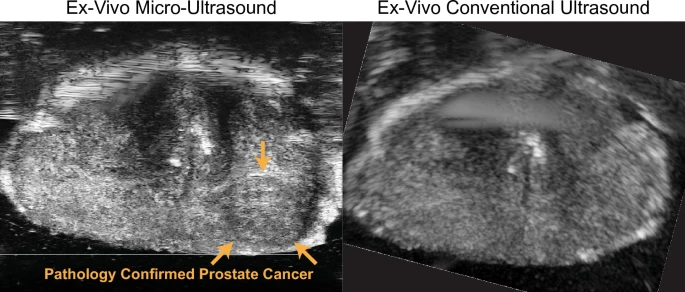
Perhaps one of the most game-changing recent developments in prostate ultrasound is the advent of micro-ultrasound, an ultra-high-frequency imaging approach. Traditional transrectal ultrasound operates in the ~6–12 MHz frequency range, which gives axial resolution on the order of 0.3–0.5 mm. Micro-ultrasound systems use much higher frequencies (often Twenty9 MHz, as with the ExactVu™ micro-ultrasound platform) to achieve resolutions around 70 microns (0.07 mm), which is about 3 times finer than standard TRUS. This high resolution can potentially allow direct visualization of prostate tumor lesions that were previously too small or subtle to see.
The concept is that micro-ultrasound might detect cancers as hypoechoic or irregular areas with much greater detail. Indeed, early clinical studies have been very promising. Micro-ultrasound can identify suspicious regions in real time during an ultrasound exam. To standardize this, a risk scoring system called PRI-MUS™ (Prostate Risk Identification using Micro-Ultrasound) was developed, analogous to the PI-RADS system on MRI. PRI-MUS uses a 1–5 scale based on micro-US findings (with 5 being highly suspicious). For example, a PRI-MUS 5 lesion might be an irregular hypoechoic area with shadowing in the peripheral zone on micro-US.
A meta-analysis in 2019 by Zhang et al. looked at micro-ultrasound’s accuracy for clinically significant cancer. It included 7 studies (769 patients) and found pooled sensitivity of 91% and specificity of 49% for micro-ultrasound in detecting clinically significant prostate cancer. The high sensitivity (~91%) is particularly notable – it rivals MRI’s sensitivity. The specificity was modest, indicating quite a few false positives (many suspicious areas on micro-US will turn out benign, hence the lower specificity). However, from a clinical standpoint, a high sensitivity is desirable in screening imaging; specificity can be managed by confirming with targeted biopsy. These results led the authors to conclude that “micro-ultrasound has superior ability to diagnose clinically significant prostate cancer” and is a convenient, cost-effective method for real-time imaging during biopsy.
Subsequent comparative studies have directly pitted micro-ultrasound against mpMRI. For instance, in a 2021 study (Guglietta et al.), micro-US and MRI were independently done and then targeted biopsies performed. They found that micro-ultrasound had detection rates on par with MRI for significant cancers. Another trial reported micro-US detected 94% of clinically significant cancers vs 90% for MRI in the same cohort. A 2024 study by Pensa et al. (Scientific Reports) used whole-mount prostatectomy specimens for ground truth and found micro-ultrasound achieved equivalent performance to retrospective MRI review for index lesion detection (91.7% vs 80%), and even detected tumor extent better, though MRI had fewer false positives per patient. Notably, prospective MRI reading in that study underperformed retrospective (73% sensitivity), highlighting both the variability of MRI and the potential consistency of micro-US. These studies suggest that micro-ultrasound can fill the gap where MRI is not done, offering an immediate, in-office alternative for high-resolution imaging of the prostate.
The practical appeal of micro-ultrasound is that it keeps the imaging and biopsy in the urologist’s office workflow. The ExactVu micro-ultrasound, for example, can toggle between conventional 12 MHz and 29 MHz modes. A urologist can scan the prostate, identify a PRI-MUS 4 or 5 region, and target that for biopsy in the same session, without needing prior MRI. This democratizes advanced imaging – no need to schedule MRI or have a radiologist interpret it; the urologist can directly visualize concerning areas. Additionally, micro-US is real-time and can scan the entire gland systematically at high resolution.
Of course, micro-ultrasound also has learning curves and limitations. The very high frequency means slightly reduced penetration – imaging the far field (anterior prostate) can be a bit more challenging in very large glands or if there’s attenuating tissue. But overall, the 29 MHz probes have been designed to visualize the full prostate adequately in most men (the gland is relatively small and accessible transrectally). There are also artifacts and normal variants that one must learn to interpret on micro-US. For instance, normal fibromuscular stroma or BPH nodules might appear different at that resolution. Training and certification programs for PRI-MUS scoring are emerging to ensure consistency.
It’s also worth mentioning that micro-ultrasound may detect some lesions that MRI misses (and vice versa). They are different modalities – micro-US excels at detecting changes in echotexture and micro-architectural disruptions, while MRI excels at detecting diffusion restriction and perfusion differences. There is growing interest in combining micro-ultrasound with MRI (if both are available). Some preliminary data indicate that using both can increase overall detection, and that micro-US could reduce reliance on systematic biopsies if MRI is done. There are scenarios where a patient’s MRI is negative (PIRADS 1-2), but micro-ultrasound finds an abnormality that yields cancer on biopsy – potentially rescuing those missed by MRI. Conversely, MRI might see something micro-US doesn’t (especially if very deep or if acoustic shadowing occurs from calcifications). Therefore, a future approach might be a sequential or combined use: e.g. do micro-US first, if something is seen target it; if micro-US is normal but suspicion remains, ensure MRI is done, etc.
In summary, high-frequency micro-ultrasound is a cutting-edge tool that dramatically enhances the resolution of prostate imaging, enabling real-time targeted detection of cancers comparable to MRI-level performance in early studies. It underscores the theme that ultrasound-based techniques are catching up to MRI as a one-stop imaging solution for prostate cancer. Micro-ultrasound is still relatively new (widely introduced in the late 2010s) and availability is not yet universal, but its adoption is growing in the U.S., Canada, and Europe. If longer-term data continue to validate its efficacy, we may see it becoming a new standard, particularly in centers without easy MRI access or for patients who prefer to avoid delays and go straight to an all-ultrasound evaluation.
Multiparametric Ultrasound (mpUS) Combination
As with MRI, where multiple sequences are combined (T2, diffusion, etc.) for better accuracy, the concept in ultrasound is to combine modalities to improve overall diagnostic performance. We have touched on this by noting that elastography and CEUS each improve detection, and Doppler adds information too. The term “multiparametric ultrasound (mpUS)” has been used to describe combining two or more of these ultrasound techniques during one exam. For example, an exam might include B-mode, color Doppler, strain elastography, and contrast enhancement together. Each modality might catch some cancers the others miss.
A 2020 systematic review by Postema et al. examined how various ultrasound methods could be combined for prostate cancer detection. It found that using multiple ultrasound parameters together improved diagnostic accuracy. In practical terms, one might first scan with gray-scale (find any hypoechoic lesions), then check those areas with Doppler (is there increased flow?), then perform elastography (are they stiff?), then inject contrast (do they enhance quickly?). A lesion that is suspicious across multiple parameters (e.g. hypoechoic, hypervascular, stiff, and shows contrast uptake) is very likely to be clinically significant cancer. In contrast, a finding that only triggers one parameter might be less concerning (for instance, a mildly hypoechoic area that is soft on elastography and no contrast uptake is probably not cancer). Combining modalities also improves confidence in negative results – if the entire gland is unremarkable on B-mode, has no focal stiffness, and no focal contrast enhancement, the chance of missing a significant cancer is quite low.
The previously cited 2024 systematic review by Jawli et al. in Cancers journal quantified some of this. It reported that while gray-scale TRUS alone had sensitivity ~38–55%, and single techniques like SWE or CEUS individually had sensitivities in the 55–88% range, a combined multiparametric ultrasound approach achieved sensitivity about 74% for overall cancer detection (with specificity ~59% in that analysis). For clinically significant cancers, mpUS sensitivity was similarly ~74%, notably higher than TRUS alone (~55%). In essence, mpUS lifted the detection closer to what mpMRI accomplishes. The trade-off was specificity; with multiple parameters you catch more true cancers at the expense of more false positives (hence specificity ~59%). But in cancer screening, a higher sensitivity is usually the priority – one can always perform a biopsy to sort out false positives.
Practically, implementing mpUS requires having ultrasound machines that support these modes (many modern high-end scanners do), as well as the contrast agent availability and expertise. It also takes a bit longer to do multiple tests in one sitting, and there is an interpretation learning curve to integrate results. But as evidence grows and if reimbursement models adapt, we may see mpUS protocols standardized in the future. For example, a possible protocol for a man with elevated PSA might be: conduct an mpUS exam with B-mode + SWE + CEUS. If the mpUS is clearly positive (suspicious areas identified), proceed to targeted TRUS biopsy of those areas. If mpUS is completely negative and clinical suspicion is low, maybe one could even avoid an immediate biopsy and instead do interval follow-up (analogous to how a negative MRI might avoid biopsy in some guidelines). Research is ongoing to validate such approaches, but early results are encouraging that a negative multiparametric ultrasound has a high negative predictive value for significant cancer.
In summary, the combination of advanced ultrasound modalities – mpUS – yields better diagnostic performance than any single modality alone. It exemplifies the principle that ultrasound can be more than just grayscale imaging; it can be a comprehensive multi-faceted imaging platform for the prostate. With mpUS, clinicians have another pathway (aside from MRI) to achieve improved cancer detection while using ultrasound as the primary imaging tool. This is especially beneficial in settings where MRI is contraindicated or not readily accessible.
Therapeutic Applications of Ultrasound in Prostate Disease
Ultrasound is not only a diagnostic tool but also plays critical roles in therapy for prostate conditions. There are two broad ways ultrasound contributes therapeutically:
- Ultrasound-Guided Interventions: Here, ultrasound imaging guides the delivery of a treatment (e.g., guiding needles or tools into the prostate). Examples include TRUS-guided brachytherapy seed placement for prostate cancer, TRUS-guided injection or aspiration procedures, and ultrasound-guided Aquablation for BPH. In these, the therapeutic effect is not from ultrasound energy itself but ultrasound ensures accurate targeting.
- Ultrasound-Based Therapies: These use ultrasound energy to ablate or treat tissue. The prime example is High-Intensity Focused Ultrasound (HIFU), which uses concentrated ultrasound beams to heat and destroy prostate tissue. Another emerging example is histotripsy, which uses focused ultrasound to mechanically break apart tissue.
We will discuss key examples with evidence for each, focusing on prostate cancer treatment and BPH interventions.
High-Intensity Focused Ultrasound (HIFU) for Prostate Cancer
HIFU is a non-invasive therapeutic modality in which high-power ultrasound waves are focused into a small point to generate intense heat (≥80–90°C) and cause immediate cell death (coagulative necrosis) in that target zone. By systematically moving the focus, an entire region (or the whole gland) can be ablated. HIFU for prostate cancer has been under development since the 1990s and has been used clinically in Europe for over two decades. In the US, HIFU was more recently approved by the FDA (in 2015) for prostate tissue ablation.
HIFU is typically delivered via a transrectal probe that houses the high-power ultrasound transducer along with imaging capability to aim it. The procedure is done under anesthesia (spinal or general) because the patient must remain still and the rectal probe is larger than a diagnostic probe. The treatment plan divides the prostate (or the targeted part of it) into small blocks, and the machine systematically sonicates each block for a few seconds to ablate it. The entire treatment can take 1–3 hours depending on prostate size and whether whole-gland or focal.
Clinical outcomes from HIFU have improved over the years and are now quite compelling for appropriately selected patients (generally those with localized prostate cancer, typically low to intermediate risk disease). For instance, a large multi-center study by Uchida et al. reported 10-year cancer-specific survival of ~97% for patients treated with HIFU, which is comparable to surgical outcomes in similar risk groups. Earlier 5-year studies showed biochemical progression-free survival around 70% for low-risk and ~50–60% for intermediate-risk disease. One 14-year study (Ganzer et al.) found 5- and 10-year biochemical failure-free survival rates of 81% and 61% respectively, in a cohort of mainly older patients with low to moderate-risk cancer. These long-term data suggest HIFU can effectively control cancer in a significant proportion of patients, though outcomes are somewhat inferior to radical prostatectomy in high-risk cases. HIFU is often positioned as a treatment for those who desire a less invasive option or are not ideal surgical candidates (e.g., older patients or those with comorbidities).
One of HIFU’s attractive features is the side effect profile. Because it is non-invasive and can be targeted (for example, hemiablation of only the cancerous side of prostate), it tends to have lower rates of complications like incontinence and erectile dysfunction compared to surgery or radiation. Reported rates of erectile dysfunction after whole-gland HIFU range ~15–40% (depending on definitions and whether nerve-sparing approaches were used), which is better than historical radical prostatectomy rates, especially in older men. Urinary incontinence rates are low, often <5–10% for significant incontinence. A multi-institutional registry noted incontinence in only ~2–3% of patients and no cases of severe grade 3 incontinence. Urethral stricture and bladder outlet obstruction can occur post-HIFU (due to tissue sloughing), with some series reporting ~5–25% needing a TURP or dilation for obstruction. There is a small risk of rectourethral fistula (<1%), but with modern techniques and limiting power near the apex, this is very rare (around 0.1–0.7%). Overall, HIFU side effects are manageable, and importantly HIFU does not preclude other treatments – if it fails, one can still do surgery or radiation as salvage (though surgery after HIFU is a bit more challenging due to scarring). Likewise, HIFU can be used as a salvage therapy itself for local recurrence after radiation, offering a second chance at cure for those patients.
From a global perspective, HIFU is more established in Europe (where devices like Ablatherm® and Focal One® from France were developed). In the U.K., HIFU is available in specialized centers and often used for focal therapy in clinical trials or registries. In the U.S., two devices (Sonablate® and Ablatherm) gained clearance mid-2010s, and usage is growing in select academic and private centers, often for focal therapy of low-risk cancers or whole-gland therapy in those who cannot have surgery. Insurance coverage is variable, which has limited widespread adoption in the U.S. so far.
In summary, HIFU is a proven ultrasound-based treatment for localized prostate cancer that can achieve long-term cancer control in many patients while minimizing morbidity. It exemplifies the versatility of ultrasound – the same energy used for imaging at low power can be used at high power to destroy tissue with precision.
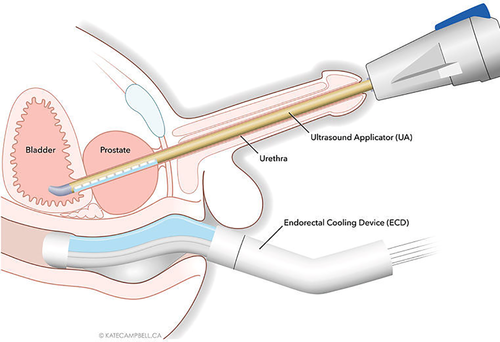
MRI-Guided Transurethral Ultrasound Ablation (TULSA)
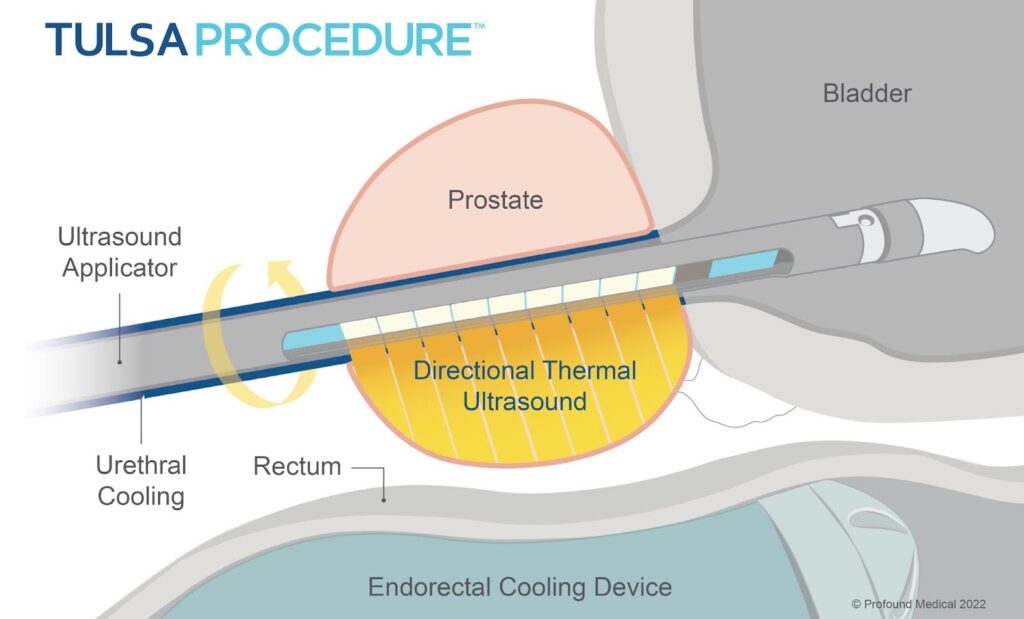
A novel variation of ultrasound therapy is the TULSA (Transurethral Ultrasound Ablation) system. Instead of a transrectal transducer, TULSA uses an ultrasound applicator inserted in the urethra (like a catheter) to emit directional high-intensity ultrasound, while the prostate is imaged in real-time with MRI for temperature monitoring and control. In essence, it’s a marriage of ultrasound therapy with MRI guidance. The procedure is done inside an MRI scanner (usually 3T), and software actively adjusts the ultrasound output based on MR thermal imaging to ensure the target tissue reaches ablative temperature while protecting surrounding structures (e.g., stopping if heat approaches the rectal wall too closely).
The TULSA-PRO system was tested in a prospective trial called TACT (Treatment of Prostate Cancer with MRI-guided Ablation) involving 115 men with localized low-intermediate risk prostate cancer. The results, published in 2020–2021, were quite encouraging. They performed whole-gland ablation in most cases (but since the device can spare the urethra and apex selectively, it can be adjusted to spare continence and potency structures). At 1-year follow-up, 96% of patients met the primary endpoint of ≥75% PSA reduction (in fact the median PSA drop was 95%). The median PSA went from 6.3 to 0.34 ng/mL, indicating near-complete ablation of prostatic tissue. On 12-month biopsy, 65% of patients had no evidence of any cancer in the prostate, and among those with residual cancer, most were lower grade (only 5% had Gleason ≥7 residual in the whole cohort). These cancer control outcomes are on par with other whole-gland therapies for that risk category.
Crucially, the side effect profile was very favorable: urinary continence was preserved in nearly all men, and 75% of men maintained or regained erectile function sufficient for intercourse by 12 months. Only 8% had any significant adverse event (Grade 3) and those were mostly transient urinary issues. The prostate volume reduced dramatically (median from 37 cc down to 3 cc) due to the ablation of tissue. These outcomes highlight that MRI-guided transurethral ultrasound can achieve effective whole-gland treatment while minimizing damage to critical structures (likely because the MR thermometry ensures controlled doses, and the ultrasound is emitted from the inside out, which may reduce heat to capsule and neurovascular bundles).
TULSA received FDA clearance for prostate tissue ablation in 2019. It’s currently available in select centers and likely will see expanded use for both primary therapy and possibly for recurrent cases (studies are exploring TULSA for radio-recurrent cancer as well). The requirement of an MRI suite and special equipment means it might be concentrated in high-volume centers. But it showcases the innovation in ultrasound therapy: using MRI to guide ultrasound ablation marries excellent imaging with precise therapy, achieving a new level of control. One could say TULSA is to HIFU what MRI-fusion biopsy is to TRUS biopsy – a hybrid approach capitalizing on the strengths of both modalities. Early salvage TULSA studies (in patients after radiation failure) also show it can effectively ablate recurrent tumors with acceptable toxicity, which expands its potential utility.
Ultrasound-Guided BPH Treatments (Aquablation and Others)
For benign prostatic hyperplasia, ultrasound has a unique role in guiding novel therapies. While not treating with ultrasound energy per se, the integration of ultrasound imaging into BPH procedures has enabled more precise and less invasive treatments:
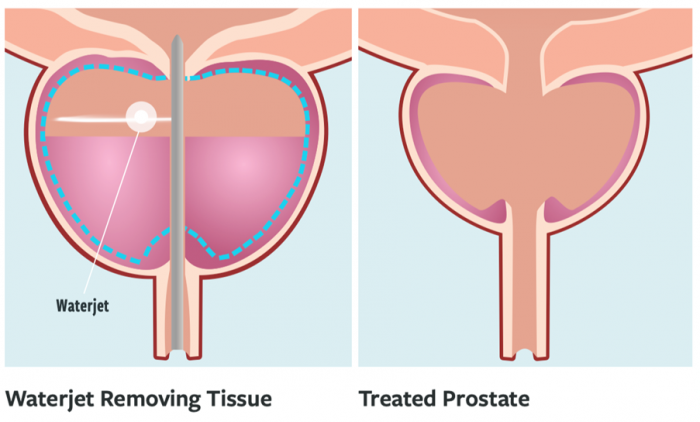
- Aquablation (Waterjet Ablation): Aquablation is a robotic procedure for BPH where a high-velocity waterjet is used to resect prostate tissue, guided in real-time by transrectal ultrasound visualization. The AquaBeam® system couples a TRUS probe (for continuous imaging of the prostate) with a robotic waterjet that is delivered via a cystoscopic sheath. The surgeon plans the tissue removal by marking the proximal and distal prostate boundaries on the ultrasound image, and then the robot automatically aims the waterjet to carve out the tissue within that plan, sparing desired areas (like the verumontanum to preserve continence and ejaculatory ducts if possible). Because ultrasound provides a clear view of the prostate lobes and capsule during resection, the system can be programmed to avoid cutting too deep (preventing capsule perforation) and to ensure adequate tissue removal where needed.
Aquablation has been proven in randomized trials to be effective. The pivotal WATER trial (2018) was a double-blind RCT comparing Aquablation to TURP for moderate-sized prostates (~50-80 cc). It showed equivalent improvements in urinary symptoms (IPSS score reduction) between Aquablation and TURP at 6 months and 1 year, meeting non-inferiority. Notably, Aquablation had a significantly lower rate of sexual side effects – in particular, ejaculatory dysfunction was much less common after Aquablation than TURP (because Aquablation can spare tissue near the ejaculatory ducts by planning around them). At 3 years, relief durability was similar, and by 5 years, outcomes remain good, with low retreatment rates in both groups. For larger prostates (80-150 cc), the WATER II trial demonstrated that Aquablation can safely and effectively debulk very large glands as well.
The advantages of Aquablation are a short resection time (since the robot can ablate tissue rapidly in a few minutes) and reduction of human variability due to automation. Bleeding is a bit more of a consideration (waterjet does not cauterize), but techniques like applying cautery at the prostatic cavity after resection have mitigated bleeding issues. The EAU guidelines have incorporated Aquablation as an alternative surgical technique for BPH, stating that evidence shows it is as effective as TURP or enucleation subjectively and objectively for tissue removal, albeit with a note about bleeding concerns. Aquablation is particularly appealing for patients who want to preserve ejaculatory function – data show a much higher rate of preserved antegrade ejaculation compared to TURP (in the WATER trial, ~90% preserved with Aquablation vs ~40% with TURP). As such, guidelines consider Aquablation an option for prostates 30–80 cc (per manufacturer recommendations) and especially in men concerned about sexual side effects.
From an ultrasound perspective, Aquablation underscores how live ultrasound guidance can be integrated into robotic surgery to improve outcomes. The TRUS not only guides but also continuously monitors during the resection, and could catch any anomalies (like if a lobe isn’t fully resected or an inadvertent capsular perforation, etc.). This synergy of real-time imaging with therapy is a theme that will likely grow.
- Other Guided Therapies: Ultrasound guides standard BPH surgeries too in some cases. For example, intraoperative transrectal ultrasound is sometimes used during simple prostatectomy (open or robotic removal of large adenomas) to delineate the bladder neck margin or to ensure complete adenoma enucleation (though this is not common). In prostate artery embolization (PAE), transabdominal ultrasound is used post-procedure to monitor changes in prostate volume over time.
Another experimental ultrasound-based therapy for BPH is histotripsy. While histotripsy has been more explored for cancer (see below), there was a pilot trial of histotripsy for BPH which indicated it can reduce prostate volume and improve symptoms without significant side effects. Histotripsy for BPH would theoretically fragment prostate tissue that is then reabsorbed or passed. This is still in investigational stages.
Histotripsy and Emerging Ultrasound Therapies
Histotripsy is a novel non-thermal ultrasound ablation technique that uses very high intensity, focused ultrasound pulses to create controlled cavitation (microbubbles) in tissue, causing mechanical destruction at a microscopic level. Unlike HIFU which relies on heat, histotripsy is sometimes called “ultrasound liquefaction.” It can homogenize the targeted tissue into an acellular slurry that the body can gradually clear. Histotripsy has been tested in preclinical models for prostate tissue ablation and even in a small human BPH study (as referenced above). The allure is that it’s non-thermal – potentially more precise in stopping at boundaries (less collateral damage from thermal spread) and possibly less painful (though still need anesthesia for prostate, presumably).
In cancer treatment, histotripsy is being explored as a focal therapy. One consideration is that the mechanical disruption might release tumor antigens more effectively (there’s interest in whether histotripsy could stimulate an immune response – essentially an immune therapy adjuvant). Trials combining histotripsy with immunotherapy for various cancers are ongoing.
While histotripsy is not yet a standard option for prostate patients, it represents the next generation of high-intensity ultrasound therapy. A review by Padilla et al. (2022) called histotripsy “the first noninvasive, non-ionizing, non-thermal ablation method” and highlighted the initial trials for BPH and other conditions. Should ongoing studies prove its safety and efficacy, we might see histotripsy systems integrated into prostate treatment in coming years.
Ultrasound in Image-Guided Focal Therapy
Beyond HIFU and TULSA, it’s worth noting that ultrasound is used to guide other focal therapies for prostate cancer, such as cryotherapy and laser ablation. In cryotherapy, multiple cryoprobes are inserted into the prostate under TRUS guidance and the freezing process is monitored with ultrasound (ice appears as an echogenic area with shadowing, and one can watch the iceball cover the tumor). Similarly, for focal laser ablation, although often done under MRI guidance, some low-resource settings have experimented with TRUS guidance for laser fiber placement.
Brachytherapy for prostate cancer (placing radioactive seeds) relies heavily on transrectal ultrasound guidance to ensure proper needle positioning throughout the gland. Treatment planning for brachy is done with TRUS images in real-time; the clinician inserts needles and seeds to match a predefined map, all visualized on ultrasound slices. This is an example where ultrasound’s real-time nature and lack of radiation make it the ideal modality for an interventional radiotherapyprocedure.
All these highlight that whether as the tool delivering therapy (HIFU, histotripsy) or as the guiding eyes for other tools (needles, lasers, waterjets), ultrasound is central to minimally invasive prostate treatments.
Ultrasound versus Other Imaging Modalities in Prostate Evaluation
When considering imaging options for prostate disease, the main modalities to compare are Ultrasound, MRI, and CT(with newer additions like PSMA PET for advanced disease staging). Each has its role, advantages, and limitations. We will provide a brief comparison, keeping in mind the context of initial evaluation and localized disease (since for metastatic disease, CT and bone scan or PET are used for staging rather than ultrasound).
- Ultrasound vs MRI (for cancer detection): MRI has become the preferred imaging for high sensitivity detection of prostate cancer lesions. As noted, mpMRI achieves sensitivities around 88–93% for significant cancers in many studies, whereas conventional TRUS is much lower. MRI also provides excellent anatomical detail for staging (e.g., seeing extraprostatic extension, seminal vesicle invasion) which ultrasound is limited in evaluating (TRUS signs of extracapsular extension have only ~40–85% accuracy and depend on operator skill). However, MRI’s downsides include high cost, variability in interpretation (one radiologist might read differently from another), and contraindications (pacemakers, some implants, claustrophobia). MRI also usually requires a separate visit and often a delay to obtain. Ultrasound, on the other hand, can be done immediately in the clinic, repeatedly if needed, and on virtually any patient. It is much cheaper and widely available. With advanced techniques (elastography, CEUS, micro-US), ultrasound’s diagnostic accuracy improves substantially, although still generally slightly behind MRI in pure sensitivity. An important difference is that ultrasound is real-time and interactive – a sonographer or urologist can move the probe, see the effect, target a needle, and get instant feedback. MRI (unless using MRI fusion or in-bore) is more static information. For biopsy guidance, ultrasound is far more convenient than trying to do biopsies in the MRI.
In many modern practices, MRI and ultrasound are used together rather than strictly competing. A patient might get an MRI to locate lesions, then ultrasound to biopsy them (fusion). Or if ultrasound is done first and shows something obvious, one might go straight to biopsy. In resource-rich settings, MRI-first is trending for new diagnoses, but ultrasound is always going to be involved in the tissue diagnosis step. In resource-limited settings or populations without MRI access, ultrasound (especially enhanced ultrasound) will remain the first-line and perhaps the only imaging. It’s worth noting that global disparities in MRI availability are significant – whereas essentially every urology practice has ultrasound. Thus, emphasizing ultrasound’s capabilities is important for global healthcare.
- Ultrasound vs CT: CT scan has a very limited role in early prostate disease evaluation. The prostate and prostate tumors are soft tissue structures that CT cannot delineate well due to poor soft tissue contrast resolution. CT cannot reliably identify an intraprostatic tumor, nor can it stage early disease with any accuracy (it may hint at gross extracapsular extension if the gland looks irregular, but that’s about it). CT is not recommended for initial diagnosis of prostate cancer. Its main use is in advanced disease – e.g., abdominal/pelvic CT to detect enlarged lymph nodes or distant metastases in high-risk patients, or to guide radiation therapy planning. Even for nodes and metastases, newer imaging like PSMA PET/CT or PET/MRI far outperforms CT in sensitivity. For benign disease, CT has virtually no role (it’s sometimes an incidental way BPH is noted if a CT was done for something else, but it’s not used for BPH work-up). Ultrasound, by contrast, excels at imaging the prostate for size and guiding interventions, without radiation exposure. So in a sense, ultrasound completely supplanted CT for any direct imaging of the prostate itself.
One niche use of CT could be in guiding calcification evaluation – if a patient has stones in the prostate or bladder that ultrasound can’t characterize, CT might show them. But this is uncommon. Transrectal or transperineal ultrasound is superior for guiding biopsies compared to CT; no one uses CT-guided prostate biopsy because it would be impractical and unhelpful.
- Ultrasound vs PSMA PET: This is a bit apples-to-oranges, since PSMA PET (often combined with CT or MRI) is used for staging and detecting metastases of prostate cancer, typically in cases with high PSA or recurrence. PSMA PET is extremely sensitive for small lymph node or bone metastases, far beyond CT or bone scan. Ultrasound cannot detect metastases except possibly large lymph nodes in the pelvis (which it’s not usually used for). So for advanced disease staging, ultrasound is not in competition – one would use CT, bone scan, or PSMA PET as appropriate. However, PSMA PET is not used for initial diagnosis of the intraprostatic tumor; it’s more for beyond the gland. There is research on high-resolution PET-ultrasound fusion to guide biopsies of PSMA-avid lesions in the prostate, but practically MRI does that job currently.
- Ultrasound vs other MRI modalities (like MR spectrography or PET/MRI): Some advanced MRI techniques exist (like spectroscopic MRI, or PET/MRI with PSMA) which can give metabolic info or combined functional imaging. These are highly specialized and not widely available. They might slightly outperform standard MRI in detection or characterization in research settings. It’s unlikely they will replace the practicality of ultrasound for biopsy guidance though.
Global perspective: In the U.S., as of the mid-2020s, we see a balanced approach: MRI is often obtained prior to biopsy in many centers (especially academic ones), but not 100% of cases (sometimes due to cost or accessibility, some urologists still proceed to TRUS biopsy particularly if suspicion is high or MRI would delay care). In community practice, many patients still go straight to TRUS biopsy if PSA is high. In Europe (e.g., UK, Sweden, Germany), guidelines and health systems have facilitated MRI-first pathways more uniformly. This has improved the quality of diagnosis but also increased reliance on radiology services. In countries with fewer MRI machines per capita, an optimized ultrasound approach could be more practical. For example, in parts of Asia, Latin America, and Africa, TRUS biopsy remains the standard initial approach due to MRI scarcity. There, training in elastography or micro-ultrasound could greatly enhance cancer detection without waiting for MRI infrastructure.
It’s also worth noting that even with MRI-first, ultrasound is not made obsolete – rather its role shifts to mainly interventional (biopsy, therapy guidance). The skills of performing and interpreting prostate ultrasound remain critical. And as we’ve outlined, new ultrasound technology can potentially reclaim more of the diagnostic role as well.
In summary, ultrasound stands as the most accessible and versatile imaging modality for prostate evaluation, whereas MRI offers higher sensitivity in detection at a higher cost and lower accessibility, and CT is largely irrelevant for local prostate imaging. A rational approach often uses them in complementary ways, but in a setting where MRI is not available, modern ultrasound techniques can serve as an effective first-line evaluation, aligning with the patient-friendly attributes of ultrasound – noninvasiveness, portability, and real-time feedback.
Conclusion
Ultrasound has been and continues to be a foundational imaging modality in the evaluation and management of prostate diseases. From the initial diagnosis of BPH or prostate cancer, through guiding biopsies, to delivering or assisting in therapy, ultrasound is involved at nearly every step of the patient journey. Advances in ultrasound technology are reinforcing its role as the first-line imaging technique for prostate assessment, especially in the initial work-up and in settings where quick, bedside evaluation is needed.
We have seen that while traditional gray-scale TRUS has limitations in cancer detection, the incorporation of new ultrasound techniques – elastography to assess stiffness, microbubble contrast to evaluate perfusion, and high-resolution micro-ultrasound for detailed anatomy – markedly improves diagnostic performance. These tools transform ultrasound from a simple picture into a multiparametric imaging platform, in many ways analogous to multiparametric MRI. The evidence from multiple peer-reviewed studies indicates that multiparametric ultrasound can detect a large proportion of clinically significant cancers, often with excellent specificity when combinations of findings are required for a positive diagnosis. For patients, this means ultrasound can often be the first test to evaluate a rising PSA, potentially obviating immediate invasive biopsy if findings are reassuring, or confidently directing a biopsy if a suspicious area is visualized.
Ultrasound’s role in prostate biopsy remains indispensable. Even as MRI is increasingly used for pre-biopsy lesion localization, the actual biopsy needle placement is almost universally done under ultrasound guidance. The development of MRI-ultrasound fusion technology is a testament to ultrasound’s continued relevance – rather than replace ultrasound, MRI complements it, and ultrasound enables MRI findings to be translated into action (targeted tissue sampling). Furthermore, techniques like transperineal ultrasound-guided biopsy are improving patient safety by minimizing infections, a refinement made possible by innovative use of ultrasound in different approaches.
On the therapeutic front, ultrasound’s versatility shines. High-intensity focused ultrasound (HIFU) has emerged as a viable curative treatment for localized prostate cancer, offering good long-term cancer control in appropriate candidates with minimal invasiveness. Newer approaches such as MRI-guided transurethral ultrasound ablation (TULSA) build on ultrasound’s therapeutic power, incorporating imaging feedback for precision. For BPH, Aquablation demonstrates how ultrasound guidance can facilitate a novel robotic surgery that rivals the gold-standard TURP in efficacy while better preserving sexual function. And in the research pipeline, histotripsy and other ultrasound-based modalities hint at a future where completely non-invasive, non-thermal ultrasound could treat prostate tissue with even greater precision.
It is important to emphasize the patient-centric advantages of ultrasound in the prostate domain. Ultrasound is typically the first imaging recommended because it’s fast, does not involve radiation, can be repeated frequently (e.g., for active surveillance monitoring or post-treatment follow-up), and has a favorable safety profile. In experienced hands, a high-quality ultrasound exam can provide a wealth of information: gland size, presence of nodules, zones of altered echogenicity, tissue stiffness distribution, and vascular patterns – all without needing a separate visit to radiology. As ultrasound machines become more advanced yet also more compact and affordable, even clinics without extensive imaging infrastructure can perform fairly advanced prostate imaging at point-of-care. This democratization of imaging is crucial in improving global prostate health outcomes.
From a sonographer’s and clinician’s perspective, the developments in prostate ultrasound mean new skill sets and opportunities. Training in elastography interpretation, contrast ultrasound administration, and micro-ultrasound PRI-MUS scoring will empower imaging specialists to detect cancers that previous generations might have missed on ultrasound. Multidisciplinary collaboration (urologists, radiologists, sonographers) in fusion biopsy or focal therapy procedures underscores how ultrasound brings together teams for patient benefit.
In conclusion, ultrasound remains the front-line imaging modality for prostate disease due to its unique combination of accessibility, real-time guidance, and now, advanced diagnostic capabilities. While MRI and other modalities have expanded the imaging arsenal, ultrasound is continuously reinventing itself to stay at the cutting edge – evidenced by elastography, CEUS, and micro-ultrasound innovations. The approach to prostate disease is increasingly one of fusion and complementarity: using each tool to its strengths. And the strength of ultrasound is that it is multifaceted: it’s our eyes during biopsy, our hands during therapy (through focused energy), and with new techniques, it’s becoming a sharper eye for diagnosis as well.
For patients, this means that the often nerve-wracking process of evaluating a worrisome PSA or deciding on a therapy can be done with minimally invasive yet highly informative ultrasound procedures, often as the first step. An evidence-based, ultrasound-first strategy – supported by confirmatory MRI or other tests as needed – may provide the optimal balance of accuracy, safety, and efficiency in prostate disease management. The peer-reviewed literature supports this evolving paradigm, and ongoing research will no doubt continue to refine ultrasound’s role. But even today, more than 50 years since it was first used for the prostate, ultrasound stands as an essential and ever-evolving ally in the fight against prostate disease.
References
- Chen FK, Abreu AL, Palmer SL. Utility of Ultrasound in the Diagnosis, Treatment, and Follow-up of Prostate Cancer: State of the Art. J Nucl Med. 2016;57(Suppl 3):13S-18S.
- Sci Rep (Pensa et al. 2024) – Evaluation of prostate cancer detection using micro-ultrasound versus MRI through co-registration to whole-mount pathology. Scientific Reports 14, 18910 (2024).
- Cancers (Jawli et al. 2024) – The Performance of Different Parametric Ultrasounds in Prostate Cancer Diagnosis: Correlation with Radical Prostatectomy Specimens. Cancers. 2024;16(8):1502.
- Liang J, Zhao Y. Ultrasound Elastography in Prostate Diseases: Current Status and Future Directions – A Review. J Clin Ultrasound. 2025 (online ahead of print).
- Zhang G et al. Micro-Ultrasound Imaging for Accuracy of Diagnosis in Clinically Significant Prostate Cancer: A Meta-Analysis. Front Oncol. 2019;9:1362
- NICE Guidance (UK 2019) – Prostate cancer: diagnosis and management. BMJ 2019;365:l2035.
- Postema et al. 2015. Multiparametric ultrasound in the detection of prostate cancer. Eur Urol 68(5):
- Ahmed HU et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22.
- Klotz L et al. Magnetic Resonance Imaging–Guided Transurethral Ultrasound Ablation of Prostate Cancer: 12-month outcomes of the TACT trial. J Urol. 2021;205(3):769-779..
- Uchida T et al. High-intensity focused ultrasound therapy for prostate cancer: five-year outcome and morbidity. Int J Urol. 2015;22(10):941-6..
- Gilling P et al. Five-year Outcomes of Aquablation vs TURP for BPH in Two Randomized Clinical Trials (WATER and WATER II). BJU Int. 2022;129(1):22-29..
- Cornud F et al. TRUS elastography and contrast-enhanced ultrasound in prostate cancer detection: a systematic review. Eur Urol. 2014;65(5):790-7.
- Pepe P et al. Shear wave elastography vs multiparametric MRI for prostate cancer detection in biopsy-naïve men: a prospective single-center study. Urology. 2017;
- Durand M et al. Multiparametric Ultrasound (B-mode, Shear Wave Elastography, Contrast Enhancement) for Prostate Cancer Diagnosis: Correspondence With Radical Prostatectomy Findings. J Urol. 2020;203(4):719-726.
- Eure G et al. Micro-ultrasound targeted biopsies for prostate cancer diagnosis: initial multi-center experiences. Prostate Cancer Prostatic Dis. 2019;22(3):466-471.
- Siddiqui MM et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. JAMA. 2015;313(4):390-397.
- Stabile A et al. Cost-effectiveness of MRI and MRI–ultrasound fusion biopsy for early detection of prostate cancer. Front Oncol. 2020;10:525706.
- Siegelman M et al. Radiologic Imaging in Prostate Cancer: Current Status and Future Perspectives. Oncol. 2019;33(11)
- Liu Y. et al. (2022) – Application of Multiple Ultrasonic Techniques in the Diagnosis of Prostate Cancer, Frontiers in Oncology 12:905087.
- Carpagnano F.A. et al. (2021) – Prostate Cancer Ultrasound: Is Still a Valid Tool?, Current Radiology Reports9(7):7.
- Liang J. & Zhao Y. (2025) – Ultrasound Elastography in Prostate Diseases: Current Status and Future Directions, J. Clinical Ultrasound (Online ahead of print, June 2025).
- Zhang M. et al. (2022) – Contrast-Enhanced Ultrasound Targeted vs Conventional Systematic Prostate Biopsy: A Meta-Analysis, Medicine (Baltimore) 101(51):e32404. –
- DuBois J. et al. (2025) – Micro-Ultrasound for Prostate Cancer Detection: A Review and Comparison with mpMRI, Tomography 11(7):80.
- Klotz L. et al. (2021) – Magnetic Resonance Imaging-Guided Transurethral Ultrasound Ablation of Prostate Cancer, Journal of Urology 205(3):769–779
- Bakavicius A. et al. (2022) – Available Evidence on HIFU for Focal Treatment of Prostate Cancer: A Systematic Review, Int. Braz J Urol 48(2):263–274
- Gilling P.J. et al. (2022) – Five-Year Outcomes for Aquablation Therapy Compared to TURP in Men with LUTS Due to BPH (WATER Trial), Canadian Journal of Urology 29(1):10960–10968.
- Posted by Bruce Gilbert
- On July 25, 2025
- 0 Comments


0 Comments